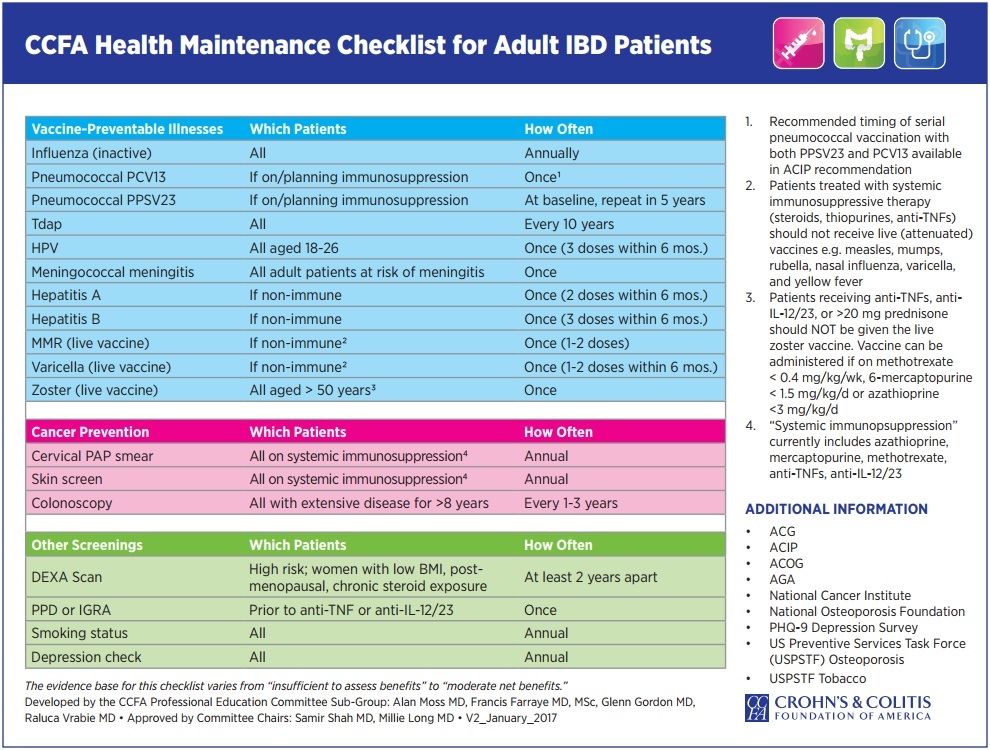
Vaccination of people with Crohn's and colitis presents several challenges. The risk of vaccine-preventable diseases and/or their complications may be increased (eg. influenza and invasive pneumococcal disease); the risk of adverse events from live vaccines (eg. MMR, MMRV, zoster, varicella, BCG, oral typhoid and yellow fever), within at least one month of immunosuppression, is increased; both the immune protection attained from previous immunisation and the response to vaccines given when immunosuppressed may be reduced. Reliable serological testing is not readily available and/or validated to measure vaccine-induced immunity for all vaccines . However, in some cases, it is useful to give additional booster vaccine or measure post-vaccination antibody titres ( eg. check VZV, measles, rubella). However, low antibody titres does not necessary reflect good immunity as other components of immunity are not easily measured (eg. T -cell and B-cell memory cells). Degrees of immunocompromise vary from insignificant to profound, and this, together with the risk of acquiring vaccine-preventable disease, should be assessed when considering a vaccination schedule. Household and other close contacts should be fully vaccinated according to current recommendations. If there is uncertainty around the level of immunocompromise and when vaccine administration may be safe, this should be discussed with the treating physician and expert advice should be sought.
Adult (≥19 years) vaccine recommendations for immunosuppressed IBD patients:
| Vaccines recommended per routine guidelines, regardless of immunosuppression | ||||||||||||||
| Influenza (trivalent inactivated vaccine) first (or new influenza vaccine strain) 2 doses 0, 4 week then annual (eg. with 2009–2010 H1N1 global pandemic seroconversion in immunocompromised improved with 2 vaccine doses | ||||||||||||||
| Tetanus (as part of dT or dTpa) every 10 years | ||||||||||||||
HPV (quadrivalent vaccine against types 6, 11, 16, and 18)
dTap=combination vaccination against diphtheria, tetanus, pertussis acellular ; HPV=human papillomavirus; MCV4=quadrivalent meningococcal vaccine; MPSV-4=quadrivalent meningococcal polysaccharide vaccine; PCV13=13-valent pneumococcal conjugate vaccine; PPSV23=23-valent pneumococcal polysaccharide vaccine; high risk country=CDC recommends HAV and typhoid |
COVID-19 Vaccination Update: 4th Dose for Severely Compromised Patients:
On 24 December 2021, the Australian Technical Advisory Group on Immunisation (ATAGI) advised:
1. People aged 18 years or older who received a 3-dose primary course due to severe immunocompromise (High dose or combination immunosuppressant medications
• High dose prednisolone)
are now recommended to receive a booster (i.e. 4
th dose) at 4 months after their 3rd dose in line with
the timing of the general population. This is expected to improve protection against symptomatic
infection, serious illness or death from COVID-19 caused by the Omicron variant.
2. Recommendations for children aged 5 to 11 years will be made in due course.
3. Antibody testing is not recommended to assess for immunity to SARS-CoV-2 following COVID-19 vaccination, including in immunocompromised individuals after a 2nd or 3rd dose as there are no
serological assays that provide a definitive correlate of immunity to SARS-CoV-2.
4. Protection from 3 primary doses with or without a booster dose in severely immunocompromised individuals may still be lower than the general population. Therefore, risk mitigation strategies such as mask wearing and social distancing should continue to be used even after receipt of a 3rd dose or
4th dose.