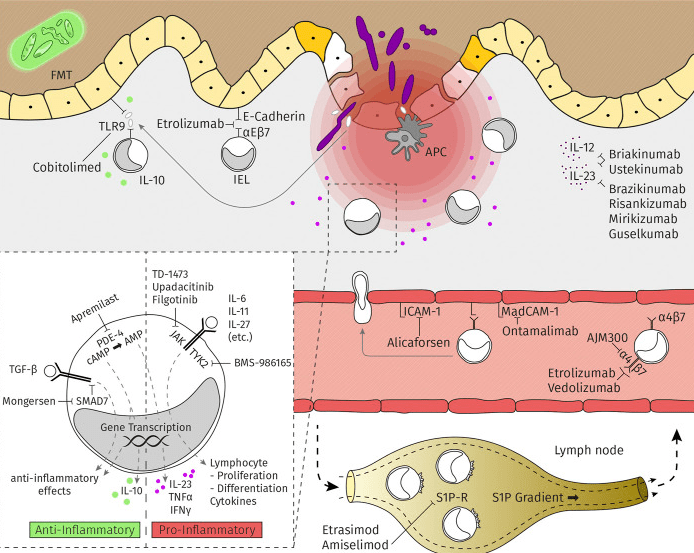
Clinical trials
Dr Doug Samuel is principal investigator currently recruiting patients for several Crohn's and colitis clinical trials. Please contact him via this website or his Crohn's and colitis clinic Tel: 9722 8794 Fax: 9722 7752.
Ulcerative colitis - 4 clinical studies recruiting now:
Ulcerative colitis [UC] is a chronic, disabling, immune-mediated disorder of the large intestine.1,2 Goals of UC treatment include achieving long-term, sustained, and durable steroid-free clinical and endoscopic remission, and preventing the need for colectomy. Anti–tumour necrosis factor alpha [TNFα] agents [eg, infliximab, adalimumab, golimumab], as well as vedolizumab, tofacitinib, and ustekinumab, are used for treatment of moderately-to-severely active UC. However, current treatment options have relatively low remission rates and/or loss of response over time and may be associated with side effects. In addition, despite the advent of novel treatments, 10–15% of patients still require a colectomy. Thus, a significant unmet need remains for novel therapies to treat this disorder:
1. GossamerBio GB004-2101
GB004 is an oral, non-biologic, gut-targeted, small molecule aiming to improve the epithelial barrier and local innate inflammatory reaction in ulcerative colitis by stabilising hypoxia inducible factor (HIF-1α), a key
transcription factor involved in the adaptive and protective cellular responses at the intersection of hypoxia and inflammation. It is not a systemimc immunosuppressant.
clinicaltrials.gov/NCT04556383
2. SER-301
This is a seamless Phase 1b multicentre study to evaluate safety, tolerability, and efficacy of SER-301 in adult participants with active mild-to-moderate UC.
SER-301 is a live microbiome therapeutic - a set of 18 live human-commensal bacterial strains representing different species administered as oral capsules. It is designed with the aim to modify the microbiome and microbe-associated metabolites in the gastrointestinal tract to modulate pathways linked to gastrointestinal inflammation and to improve epithelial barrier integrity in patients with ulcerative colitis.
• SER-301 Part 1 is a liquid formulation of bacterial spores containing 10 strains delivered in 2 oral capsules. Part 1 contains 5.6 x 10^6 CFU.
• SER-301 Part 2 is a dry powder formulation of vegetative bacteria containing 8 strains delivered in 2 oral capsules. Part 2 contains 4.5 x 10^7 CFU.This study is composed of two study parts, aiming for an operationally seamless transition in between. Both study parts share objectives of safety, tolerability, and engraftment measures.Participants will be enrolled into either Part 1 or Part 2 of the study, but not both. This record describes Part 1 only.Part 1 is open-label. Approximately 15 participants will be enrolled in 2 sub-cohorts to receive 10 weeks of once-daily open-label induction treatment with SER-301 following 6 days of vancomycin pre-conditioning (125mg oral capsules, 4 times daily).
• Part 1A: 5 participants will be dosed with SER-301 after vancomycin pre-conditioning. If no significant safety concerns are reported after all 5 participants have completed pre-conditioning and 7 days of SER-301 dosing, enrollment in Cohort 1B will proceed.
• Part 1B: The remaining 10 additional participants will be dosed with SER-301 after vancomycin pre-conditioning. If no significant safety concerns are reported after all 10 participants have completed pre-conditioning and 7 days of SER-301 dosing, enrollment in Cohort 2 will proceed.Adherence will be monitored throughout the study. All remaining drug should be returned by participants to the clinical site where drug accountability, including capsule count to monitor compliance, will take place and participants will also be asked to complete a diary where they will record daily symptoms.
3. Arena - APD334-302
ELEVATE UC 12: Etrasimod Versus Placebo as Induction Therapy in Moderately to Severely Active Ulcerative Colitis
Sphingosine 1-phosphate [S1P] receptor modulation has been investigated as a potential treatment pathway for a number of immune-mediated conditions and has been widely used in multiple sclerosis over the past decade. The interaction of S1P with S1P receptors 1 [S1P1] through 5 [S1P5] modulates a wide range of biological functions, including lymphocyte trafficking and endothelial barrier integrity. The S1P receptor modulators fingolimod, siponimod, and ozanimod have been approved by regulators for treatment of multiple sclerosis, and ozanimod has also been studied for treatment of moderate-to-severe UC for up to 32 weeks.
Etrasimod is an oral, selective sphingosine 1-phosphate (S1P1, S1P4, and S1P5) receptor modulator in development for treatment of immune- and inflammatory-mediated diseases including ulcerative colitis. Etrasimod 2 mg provided significant benefit compared with placebo and was generally well tolerated in the OASIS study [NCT02447302]. In an open-label extension [OLE] study [NCT02536404] evaluated, for up to an additional 34 to 40 weeks [46 to 52 weeks total], the safety and efficacy of once-daily etrasimod 2 mg in achieving and maintaining clinical response and/or remission in patients who completed OASIS demonstrated a favourable safety profile. Most patients with clinical response, clinical remission, or endoscopic improvement at Week 12 maintained that status to end of treatment. There were no treatment-related serious infections, and no patient had an infection of severity grade ≥ 3. The most commonly reported TEAEs in patients receiving any etrasimod 2 mg in the OLE, occurring in ≥ 10% of patients, were worsening UC (21/112 [19%] patients) and anaemia (12/112 [11%] patients]). No patients discontinued due to low lymphocyte count.
clinicaltrials.gov - 12 week study
4. Arena -APD334-303
An Extension Study for Treatment of Moderately to Severely Active Ulcerative Colitis (ELEVATE UC OLE
The purpose of this open-label extension (OLE) study is to evaluate the safety and efficacy of etrasimod in participants with moderately to severely active ulcerative colitis (UC) who previously received double-blinded treatment (either etrasimod 2 mg per day or placebo) during participation in one of two Phase 3 double-blinded, placebo-controlled studies (either Study APD334-301 or APD334-302).
Crohn's disease - 2 clinical studies
Crohn’s disease (CD) is a chronic intermittent and often progressive inflammatory disorder of the gastrointestinal tract with a variable presentation. It frequently requires long term maintenance therapy to control disease activity. Many of these current therapies have important limitations including inadequate efficacy and undesirable adverse events (AEs). Although corticosteroids commonly succeed to induce remission their long term use is associated with significant AEs and thiopurine and methotrexate have similar limitations in many. Biological agents including anti–tumour necrosis factor alpha [TNFα] agents [eg, infliximab, adalimumab], and more recently vedolizumab and ustekinumab, are used for treatment of moderately-to-severely active CD. For CD, therapeutic efficacy of biologicals tends to be substantially lower when patients have failed prior biological treatment.
Over two‐thirds of CD patients discontinue anti‐TNF‐treatment over time due to primary or secondary non-response or adverse effects. For example with infliximab, the first of the anti-TNFs available in Australia, failure to initially respond to IFX therapy, known as primary non-response, has been noted to occur in as many as 29–41% of patients with luminal CD and 31% of patients with fistulizing CD. The loss of a previously achieved clinical response, known as secondary non-response, occurs by week 54 in 61% of patients receiving IFX at 5mg/kg every 8 weeks and in 42% of patients receiving 10mg/kg every 8 weeks.
While it is not fully known why primary and secondary non-response occurs in CD, one hypothesis is that free anti-TNF concentrations become inadequate due to increased drug clearance from multiple causes including the presence of antibodies to infliximab or adalimumab, and that some patients’ disease may be mediated through non-TNF pathways. Free anti-TNF levels are associated with improved clinical, laboratory, and endoscopic responses in a number of (but not all) observational and prospective studies. One strategy for addressing primary and secondary non-response to IFX is to escalate IFX dosing or switch to another agent based on serum IFX (or ADA) and ATI assays. In the appropriate pharmacokinetic milieu (subtherapeutic serum IFX and absent ATIs) when standard dose IFX is inadequate, doctors escalate therapy either by increasing the dosage administered or decreasing the interval between infusions rather than switching to adalimumab or choosing a second biological. Retrospective studies have shown dose escalation response rates of 66–96% among all patients who have lost response to doses of 5mg/kg every 8 weeks, with one study observing the response rate to IFX escalation to be 88% in patients without antibodies to infliximab [ATI] compared with 33% among those with ATIs. The sustained response rate to IFX intensification at 12 months is estimated to be 47%.
One retrospective study from Mount Sinai looked at their 86 patients (27% of their IFX infusion cohort) on high dose IFX (defined as 10mg/kg every 4 to 7 weeks, or 15 to 22.5mg/kg every 4 to 8 weeks ie. maximum dose 22.5mg/kg every 4 weeks. 1/3 had "therapeutic" infliximab level>3 at start of high dose therapy and 1/3 had ATIs. An increase in IFX level after or ATIs did not predict response). Serious adverse events including infections occurred in 9 of the 86 patient. Acute and delayed infusion reactions which didn't respond to premedication occurred in 2 of the 15. The rates of clinical response after escalating to an HD IFX regimen [84.9% and 62.3% at the early and late time periods, respectively] closely parallel the response rates of 66–96% seen in other studies examining standard escalation from 5mg/kg every 8 weeks to 5mg/kg every 4 weeks or to 10mg/kg every 8 weeks.
For those who then choose a second or third biological agent, the 12 month corticosteroid‐free efficacy is often disappointing: for vedolizumab 27-32% and for ustekinumab 46% if vedo-naive and 26% if vedo-failure. The original ustekinumab UNITI1/2 landmark studies showed clinical response and remission of 58% and 40% if anti-TNF naive and 38% and 20% if anti-TNF failure at 8 weeks with 50% of this effect shared with placebo. A further 10%, slower responders, were in remission at the end of 16 weeks. When the best responders from UNITI1/2 studies were followed for 5 years, only 20% who had failed anti-TNFs and 25-40% who had been TNF-naive were still in steroid-free clinical remission.
NONPHARMACOLOGICAL THERAPIES
Along with patient interest, there is increasing scientific and medical recognition of the role of diet in IBD pathogenesis and treatment. Therapeutic diets involve exclusion of foods hypothesised to contribute to dysbiosis and negatively affect intestinal barrier, cell tight junctions, mucous membrane and immune function and others to optimise short chain fatty acids. Exclusive enteral nutrition (EEN), where a patient’s entire nutrition needs are met via a liquid formula diet, has been shown superior to corticosteroids in inducing remission in paediatric CD. In adults, where palatability clashes with acceptability, it's role is limited to inducing remission after a new CD diagnosis or for preoperative optimisation of CD patients to reduce risk of complications. The CD exclusion diet (CDED) involves daily consumption of lean protein, starches, and fibres to act as substrates for short-chain fatty acid (SCFA)-producing bacteria. SCFAs, such as butyrate, act as energy sources for colonic epithelium. In one study CDED plus partial enteral nutrition (PEN) showed better tolerability and equivalent Week 6 corticosteroid-free remission compared to EEN in paediatric Crohn's. In the following 6 weeks, CDED plus PEN maintained remission while transfer to a free diet plus PEN did not. More diet research is needed.
Thus, a significant unmet need remains for novel therapies to treat this disorder. With strict in‐ and exclusion criteria, one‐third of CD patients in standard care may be eligible for phase 2/3 clinical trials:
1. DIONE 0173
A Phase 2 multi-center, randomized, double blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of 12 weeks of induction therapy with TD˗1473 in subjects with moderately-to-severely active Crohn's disease. This study includes 3 phases: Screening, Induction, and Active Treatment Extension (ATE). The Induction phase of the study is a randomized, double blind, placebo controlled, parallel group study evaluating 2 oral dose levels of TD-1473 compared to placebo for 12 weeks in subjects with moderately to-severely active CD. Subjects who complete Induction will continue to receive TD-1473 in the ATE, for up to 48 additional weeks.
TD-1473 is an oral pan-JAK inhibitor designed to inhibit the JAK family of enzymes locally in the GI mucosa while minimizing systemic exposure through limited oral absorption and rapid systemic clearance.
clinicaltrials.gov - 12 week induction, 48 week active treatment
2. Celgene
Induction Study #1-3 of Oral Ozanimod as Induction Therapy for Moderately to Severely Active Crohn's Disease
clnicaltrials.gov _ Phase 3 Ozanimod study #1
clincaltrials.gov - Phase 3 Ozanimod study #2
Phase 3, randomized, double-blind, placebo-controlled study to explore the effect of oral ozanimod as an induction treatment for subjects with moderately to severely active Crohn's Disease.
Eligibility:
You may be able to take part in this ozanimod study if you:
- are 18 to 75 years of age
- have been diagnosed with active Crohn's disease confirmed by endoscopy (a procedure where a doctor looks inside your digestive tract using a device called an endoscope).
- currently have symptoms associated with moderately to severely active Crohn's disease, and
- have not improved on or have not been able to tolerate, at least one prior Crohn's disease medication†
†Eligible prior medications include corticosteroids, immunomodulators or biologic therapy (e.g., ustekinumab, TNFα antagonists or vedolizumab).
Exclusion Criteria: The presence of any of the following will exclude you from participation in the study:
- Diagnosis of ulcerative colitis, indeterminate colitis, radiation colitis, or ischemic colitis or known strictures or stenosis leading to symptoms of obstruction.
- Current stoma, connection of your ileal and anal pouch, symptomatic fistula, or need for ileostomy or colostomy
*Other inclusion/exclusion criteria apply
Study Details:
Study Description
YELLOWSTONE Induction Study (RPC01-3202)
- This study is designed to determine the safety and effectiveness of the oral investigational study drug, ozanimod, versus a placebo (an inactive substance) in achieving symptom remission in patients with active Crohn's disease symptoms. An induction study is the first in a series of studies. Participation in this clinical study is expected to last 12 weeks (3 months).
- Depending on response and the study doctor's recommendation, participants may have the opportunity to continue participation in the YELLOWSTONE Maintenance RPC01-3203 or YELLOWSTONE Open-Label Extension Study (RPC01-3204). An open label extension study means you may be able to continue taking the investigational study drug, if you qualify and choose to participate.
Learn more at www.crohnsstudies.com.
Description of Treatment or Intervention (Mechanism of Action)
Ozanimod is thought to act on the immune system by encouraging certain types of white blood cells called lymphocytes, which include T cells, to stay in the lymph nodes and other places in the body, thereby keeping them away from sites of inflammation.
Lymphocytes, which act as the body's mechanism to fight off invaders, are responsible for initiating the immune response. However, in Crohn's disease, lymphocytes misread the inflammation caused by the disease as an area where their help is needed.
Patient Participation Requirements
- Visits to the trial site will be required approximately every 4 weeks for the Induction study.
- A variety of assessments such as lab tests, ECGs, vital signs, physical exams, eye exams, endoscopies and pulmonary function tests will be performed.
- Participants will need to complete some questionnaires during visits to the trial site, and to keep a study diary (e-diary) daily.
- If you are living with active Crohn's disease and experiencing symptoms, you may want to discuss this study with a participating trial site where the study will be explained to you in greater detail and you will be given an opportunity to ask any questions.
Possible Risks & Side Effects
Ozanimod is still being studied, so not all of its side effects are known. In the phase 2 clinical trial, the most common serious adverse events in two or more patients were Crohn's disease flare, fistulizing disease, intestinal obstruction and abdominal abscess. If you qualify for the trial, more information will be given to you by the study site doctor or a member of their staff.
recently closed studies:
1. Faecal Microbiota Transplantation in Ulcerative Colitis (FOCUS)
http://clinicaltrials.gov/ct2/show/NCT01896635
The FOCUS Study is the exciting new Australian study of “Faecal Microbiota Transplantation” (FMT) for patients with active mild to moderate ulcerative colitis (despite medications). It is a placebo controlled study, consisting of one colonoscopy treatment followed by patient-administered enemas. Patients who fail to improve can get open-label FMT after they conclude their study period. The FOCUS Study will run only till the end of this year
Contact: Dr Samuel, our study coordinator Sudarshan Paramsothy on focusstudygroup@gmail.com or my Crohn's and colitis clinic.
The FOCUS Team: Michael Kamm, Hazel Michell, Thomas Borody, Alissa Walsh, Johan van den Bogaerde, Doug Samuel, Susan Connor, Watson Ng, Rupert Leong, Enmoore Lin, Sarah Finlayson and Sudarshan Paramsothy.
Please contact one of our team for access to password protected information sheets:
FOCUS Participation Information and Consent Form
Invitation
You are invited to participate in a research study into the efficacy and safety of regular “healthy donor” faecal microbiota administration as a treatment for chronic active ulcerative colitis.
“Efficacy” refers to the effectiveness of a therapy or the ability of a therapy to
successfully produce the desired result – in this case, reducing the severity of
ulcerative colitis and associated symptoms.
The study is being conducted by Dr Douglas Samuel, Gastroenterologist at Bankstown-Lidcombe Hospital. The study is part of a national collaborative study coordinated by Australian researchers into ulcerative colitis. Other sites include St Vincent's Hospital, Darlinghurst with Dr Alissa Walsh.
Before you decide whether or not you wish to participate in this study, it is important for you to understand why the research is being done and what it will
involve. Please take time to read the information carefully, and if you wish, discuss
it with your family, friends and your GP. The study doctor will be happy to discuss
it with you and explain any words or information you do not fully understand. Ask
questions if anything is unclear or if you would like more information. Take time to
decide whether or not you wish to take part.
What is the purpose of this study and what is Faecal Microbiota Transplantation (FMT)?
The purpose of this study is to investigate whether faecal microbiota transplantation (FMT), a therapy comprised of a faeces mixture provided by healthy human donors is an effective and safe treatment for chronic active ulcerative colitis (UC) unresponsive to standard medical therapy.
Ulcerative colitis is a chronic bowel condition characterised by inflammation of the large intestine and rectum. Associated symptoms typically include, diarrhoea, rectal bleeding, urgency to defecate and incontinence.
While the exact cause of UC is unknown, there is now compelling evidence that the gut bacteria play a central role in the development of UC, by stimulating an inappropriate immune system response. Current standard treatment for ulcerative colitis involves medications that alter the immune system response, but these only work in a proportion of patients with UC and can have associated side effects.
This in turn has directed research efforts into alternative therapies that look at restoration or replacement of a patient’s gut bacteria with gut bacteria obtained from a healthy donor with the aim of correcting any abnormalities and restoring missing components. The human bowel contains a complex population of gut bacteria. These gut bacteria and the chemicals they produce affect the body and these effects can have both positive and negative impacts on a person’s health. The normal or natural human gut bacteria protect us from pathogenic or “bad” bacteria.
Faecal microbiota transplantation (FMT) involves the infusion of healthy human donor faeces via colonoscope or enema into the “sick” diseased bowel of the patient. The use of healthy human gut bacteria in the faeces appears to be the most “complete probiotic” treatment possible, capable of eradicating “bad bacteria” and supplying “good” bacteria for re-colonisation and restitution of normal gut bacteria.
There has been recent evidence published in the medical literature demonstrating the effectiveness of FMT in treating Clostridium difficile infection (CDI), a gastrointestinal infection caused by a bacteria, that can often be unresponsive to multiple courses of antibiotic therapy. Given that FMT is capable of successfully treating unresponsive CDI colitis, FMT may have a role in other diseases where bacteria play a role in disease development, such as UC. Restoration of healthy gut bacteria may represent an effective therapy for UC.
There have been a few individual patient reports and a small series of reports suggesting possible benefit of FMT for UC, but as of yet there have been no published clinical trials to confirm this. We are conducting a trial to determine the benefit and safety of regular FMT therapy in UC. We also aim to collect and analyse faecal samples from healthy donor mixtures along with study patients to investigate changes in the gut bacteria of UC patients undergoing FMT that may be associated with benefit.[/vc_toggle][vc_toggle title="Why have I been invited to participate in this study?" open="false"]You are eligible to participate in this study because you have chronic active mild or moderate Ulcerative Colitis of >3 months duration currently not in remission and satisfy the selection criteria for study participation.
What if I don’t want to take part in this study, or if I want to withdraw later?
Participation in this study is voluntary. It is completely up to you whether or not you participate. If you decide not to participate, it will not affect the treatment you receive now or in the future. Whatever your decision, it will not affect your relationship with the staff caring for you.
New information about the treatment being studied may become available during the course of the study. You will be kept informed of any significant new findings that may affect your willingness to continue in the study.
If you wish to withdraw from the study once it has started, you can do so at any time without having to give a reason. There are no consequences that will arise from withdrawing from the trial.
Participation is entirely free and voluntary and patients are free to withdraw at any stage for any reason. Study withdrawal will not influence your relationship with the study investigators or the quality of care that you will receive. Patients will be able to access and complete the remainder of a total of 8 weeks of active FMT study enema therapy in an open label manner post study withdrawal if they so wish.
However, it may not be possible to return your samples to you or withdraw your data from the study results if these have already had your identifying details removed.
What does this study involve?
If you agree to participate in this study, you will be asked to sign the Participant
Consent Form.
Individual patient participation duration will consist of 8 weeks of study therapy and then follow up in a further 8 weeks time.
The treatment being investigated in this study differs from the standard treatment offered in this institution because of its use of faecal microbiota transplantation(FMT) with healthy donor. FMT is attracting increasing global interest as an agent that may be useful in treating ulcerative colitis by restoring normal healthy gut bacteria, abnormality of which is believed to contribute to the disease.
This trial is a randomised, double blind, placebo controlled study comparing active
donor FMT therapy with placebo therapy
‘Randomised trial’: Sometimes doctors don’t know the best way of treating patients with a particular condition so comparisons need to be made between different treatments. To do this, study participants are put into groups and given different treatments, and the results are compared to see whether one treatment is better. To ensure the groups are similar to start with, a computer allocates each study participant into a group randomly, like the flip of a coin. Neither the doctor nor the study participant can decide which treatment the participant receives.
‘Blind trial’: In a “blind trial” the study participants do not know which treatment group they are in. If the trial is “double blind”, neither the doctor nor the study participant knows which treatment the participant is receiving (although, if the doctor needs to find out, he/she can do so).
‘Placebo’: A placebo is a dummy treatment that looks like the genuine medicine but contains no active ingredient.
If you agree to participate in this trial, you will be asked to undergo a medical review with medical history and physical examination to ensure you satisfy the study selection criteria. You will then be invited to participate in the study after thorough explanation of the study requirements, answering of any questions or concerns by a study investigator and completion of the accompanying informed consent sheet.
Recruited participants will undergo baseline blood and faecal tests as part of screening and assessment of disease activity. They will then be randomly allocated to either active FMT therapy or placebo for 8 weeks. Study subjects will be blinded i.e. they will not know which treatment arm they are randomly allocated to, as will be the study investigators.
Please contact us if you want more details of the 8 week study therapy. Individuals randomised to placebo therapy who are not in remission after the 8 week study period will be eligible to receive 8 weeks of open label (“unblinded”) active FMT therapy. 8 weeks post completion of final study therapy (whether blinded or open label /unblinded), study subjects will be reviewed for a final time for follow up on their clinical progress, assessment of disease activity and collection of final blood and faecal samples for study analysis.
Regular scheduled study monitoring calls and face to face visits will occur on an alternating weekly basis to assess patient response/disease activity, compliance and any side effects or other complaints. At predetermined time points on a monthly to second monthly basis, further faecal and blood samples will be collected for monitoring of disease activity and microbial analysis.
Participating in the trial will require some restrictions on your lifestyle during the study.
Recruited patients will be instructed to minimise alcohol intake to ≤ 2 standard drinks/day whilst they are participating in the study.
While no specific dietary restrictions will be imposed, recruited patients will be advised to be judicious in the choice of food consumed during the study period to minimise the risk of food poisoning. Subjects are asked to exercise reasonable caution to avoid food poisoning during the study period, such as refraining from eating seafood or raw/uncooked food such as sushi, takeaway etc.
Overseas or interstate travel will also need to be restricted during the study period to ensure compliance with study enema therapy 5 days per week.
Recruited patients are precluded from any antibiotic or probiotic therapy during the study period or in the preceding 1 month and 3 months respectively.
In addition, the researchers may request access to your medical records to obtain information regarding your medical background and ulcerative colitis disease course (including prior medications / therapies) relevant to the study.[/vc_toggle][vc_toggle title="Are there risks to me in taking part in this study?" open="false"]All medical procedures involve some risk of injury. In addition, there may be risks associated with this study that are presently unknown or unforeseeable. In spite of all reasonable precautions, you might develop medical complications from participating in this study.
There is minimal data to date with regards to FMT therapy for ulcerative colitis. Most published literature related to FMT therapy involves treatment of Clostridium difficile colitis, a gut infection. Such studies involving FMT administration to date have not demonstrated any serious adverse events. The known side effect profile and complications associated with FMT therapy is minimal.
A minority of patients (approximately 1 in 3 to 4 people) note some irregularity of bowel movements and excessive flatulence / bloating during the first couple of infusions but these symptoms are minor and usually resolve. Nonspecific abdominal discomfort has also been reported which again is usually minor and self-limiting.
There is a theoretical risk of infectious disease transmission from the donor (including but not limited to hepatitis, HIV, other viruses and parasites) associated with FMT. Donors of the stool used to constitute the FMT will be rigorously screened as per internationally published and accepted guidelines to minimise potential transmission of infectious disease. To date there have been no published cases or documented reports regarding transmission of infective agents / diseases by FMT in the medical literature. There is also a small risk of rupture / perforation of the rectum or bowel at the time of endoscopy (roughly 1 in 1000 cases), that may necessitate surgery.
Theoretically there is an extremely low likelihood this may also occur with enema self administration as the enema tip is inserted into the rectum.
Study participation will require the administration of either active donor FMT or placebo thoughout the study period which can be associated with the inconvenience of poor retention, urgency and incontinence, usually limited to the first couple of infusions. This may necessitate the use of incontinence pads. Repeated administration can also occasionally be associated with small tears or “micro-fissures” around the anus.
It is important that women participating in this study are not pregnant and do not become pregnant during the study as the effect of the study therapy of donor FMT on an unborn baby is unknown. If you are a woman of childbearing age and there is any possibility that you are pregnant, the researchers will need to perform a urine pregnancy test before you start in the study. Women of childbearing age will have to practice an effective form of contraception during the study.
Will I benefit from the study?
This study aims to further medical knowledge and may improve future treatment of ulcerative colitis, however it may not directly benefit you.
All study patients will have access to active study therapy with donor faecal enemas (either blinded or open label post blinded study therapy). This will ensure all study participants will have the capacity to benefit from trial participation if any study therapy efficacy is demonstrated.
Will taking part in this study cost me anything, and will I be paid?
Participation in this study will not cost you anything. This study is an investigator initiated project dependent on limited grant funding to cover the costs of the trial. The study investigators / doctors are not paid and provide their time and services for free. The study participants will be provided with the study treatment and care for free. Study participants will not be paid for participating in the trial.
What should I do if I want to discuss this study further before I decide?
When you have read this information, the researcher Dr Douglas Samuel and/or Dr Sudarshan Paramsothy will discuss it with you and answer any queries you may have. If you would like to know more at any stage, please do not hesitate to contact him/her on (02) 9722 8794.
2.AMG 181 Phase 2 Study in Subjects With Moderate to Severe Ulcerative Colitis
http://clinicaltrials.gov/show/NCT01694485
AMG 181 is a human anti-α4 β7 antibody given by subcutaneous injection. This randomized, double blind, placebo controlled study is looking to help patients with moderate to severe ulcerative colitis. AMG 181 targets the α4 β7 integrin heterodimer, blocking its interaction with mucosal addressin cell adhesion molecule-1 (MAdCAM-1), the principal ligand that mediates α4 β7 T cell gut-homing. At the end of the double blind period, all subjects will be eligible to receive open label AMG 181.[/vc_column_text][vc_separator color="grey" style="" el_width=""][/vc_column][/vc_row][vc_row][vc_column width="1/1"][vc_column_text]
3. A Study of Safety and Effectiveness of JNJ-54781532 in Patients With Moderately to Severely Active Ulcerative Colitis
http://www.clinicaltrials.gov/show/NCT01959282
JNJ-54781532 is an oral JAK 1/3 inhibitor. Tofacinib, an alternative JAK 1/3 inhibitor, is approved for use in rheumatoid arthritis and results of phase III studies in ulcerative colitis are pending. This randomized, double-blind, placebo-controlled, parallel group, dose-response study evaluates several doses of JNJ-54781532 in patients with moderately to severely active ulcerative colitis (UC). The JAK-STAT pathway is an essential part of the human gut's immune system. [/vc_column_text][vc_separator color="grey"][/vc_column][/vc_row][vc_row][vc_column width="1/1"][vc_column_text]
4. A Study Comparing the Efficacy and Safety of Etrolizumab With Adalimumab and Placebo in Patients With Moderate to Severe Ulcerative Colitis in Patients Naive to TNF Inhibitors
http://clinicaltrials.gov/show/NCT02163759
This Phase III, double blind, placebo and active comparator controlled, multicenter study will investigate the efficacy and safety of etrolizumab in induction of remission in patients with moderately to severely active ulcerative colitis (UC) who are naïve to TNF inhibitors and refractory to or intolerant of prior immunosuppressant and/or corticosteroid treatment.
5. A Study of the Efficacy and Safety of Etrolizumab in Ulcerative Colitis Patients Who Are Refractory to or Intolerant of TNF Inhibitors.
http://clinicaltrials.gov/ct2/show/NCT02100696
For a more thorough search of clinical trials in Australia and around the world, visit the website www.clinicaltrials.gov. Additional portals to look for trials can also be found at the Australian Clinical Trials, World Health Organization and the IFPMA sites.
Past studies at Bankstown Crohn's and colitis clinic:
COMPLETED
2015 –
Genentech – ETRO CD
Protocol GA29144: A Phase III, Randomized, Double-Blind, Placebo-Controlled, Multicenter Study To Evaluate The Efficacy And Safety Of Etrolizumab As An Induction And Maintenance Treatment For Patients With Moderate To Severely Active Crohn’s Disease
2015 –
Genentech – ETRO CD
Protocol GA29145: An Open–Label Extension and Safety Monitoring Study of Patients with Moderately to Severely Active Crohn’s Disease Previously Enrolled in the Etrolizumab Phase III Protocol GA 29144
2016 – 2018
Celgene – GED-0301
Protocol GED-0301-002 : A Phase 3, Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Investigate the Efficacy and Safety of Mongersen (GED-0301) for the Treatment of Subjects with Active Crohn’s Disease
2016 – 2018
Celgene – GED-0301
Protocol GED-0301-004 : A Phase 3, Long-Term Active Treatment Extension Study of Mongersen (GED-0301) in Subjects with Active Crohn’s Disease
2015 – 2018
RedHill BioPharma Ltd/ InSymbiosis
Protocol No : RHB-104-01 A Phase III, Double-Blind, Randomized, Placebo-controlled, Multicenter, Parallel Group Study to Assess the Efficacy and Safety of Fixed-dose Combination RHB-104 in Subjects with Moderately to Severely Active Crohn’s Disease
2008-2015
CDP – UCB Celltech (PPD)
Protocol Number C87088 A Phase IIIb, multinational, open-label label, follow-on trial to C87085 designed to assess the long-term safety of certolizumab pegol, a pegylated Fab’ fragment of a humanised anti-TNF-alpha monoclonal antibody, administered subcutaneously at weeks 0, 2 and 4 and then every 4 weeks thereafter, in subjects with moderately to severely active Crohn’s Disease who have participated in study C87085
2009-2014
MLN0002 – Millenium (Quintiles)
Protocol No C13008 A Phase 3, Multiple Dose, Open-label Study to Determine the Long-Term Safety & Efficacy of MLN0002 in Patients with Ulcerative Colitis & Crohn's Disease
2012- 2014
CCX114643 (GSK) - Vercirnon
Protocol No CCX 114643: A Randomised, Double-blind, Active Treatment Study to Induce the Clinical Response and/or Remission with GSK1605786A in Subjects with Moderately-to-Severely Active Crohn’s Disease
2011- 2014
CCX114151 (GSK) - Vercirnon
Protocol No CCX 114151: A Randomised, Double-blind, Placebo-Controlled Study to Investigate the Efficacy and Safety of GSK1605786A in the Treatment of Subjects with Moderately-to-Severely Active Crohn’s Disease
2011- 2014
CCX114157 (GSK) - Vercirnon
Protocol No CCX 114157: A 52 week Randomised, Double-blind, Placebo-Controlled Study to Investigate the Efficacy and Safety of GSK1605786A in the Maintenance of Remission in Subjects with Crohn’s Disease
2011-2014
CCX114644 (GSK) - Vercirnon
Protocol No CCX 114644: An Open-Label Extension Study to Assess the Safety of GSK1605786A in Subjects with Crohn’s Disease
2010-2013
POCER – University of Melbourne
Protocol POCER Post-Operative Crohn's Disease Endoscopic Recurrence “POCER” study: Endoscopic Guided Therapeutic Intervention& Determination of Cause
2011-2012 Lead Site
MLN0002 – Millenium (Quintiles)
Protocol C13011: A Phase 3, Randomised, Placebo-controlled, Blinded, Multicenter Study of the Induction of Clinical Response and Remission By Vedolizumab in Patients With Moderate to Severe Crohn's Disease
2009-2012
MLN0002 – Millenium (Quintiles)
Protocol No C13007 A Phase 3, Randomized, Placebo-Controlled, Double-Blind, Multicenter, Multiple Dose Study to Determine the Efficacy & Safety of MLN0002 for the Induction& Maintenance of Clinical Response & Remission in Patients with Active Crohn's Disease
2009-2011
Ustekinumab – Centocor
Protocol C0743T26 A Phase 2b, Multicenter, Randomized, Double-blind, Placebo-controlled, Parallel-group Study to Evaluate the Efficacy and Safety of Ustekinumab Therapy in Subjects with Moderately to Severely Active Crohn's Disease Previously Treated with TNF Antagonist Therapy
2008-9
CDP – UCB Celltech (PPD)
Protocol Number C87085 A Phase IIIb, multinational, randomized, double-controlled trial to assess the efficacy and safety of certolizumab pegol, a pegylated Fab’ fragment of a humanised anti-TNF-alpha monoclonal antibody, administered subcutaneously at weeks 0, 2 and 4 in subjects with moderately to severely active Crohn’s Disease.
2006-9
Traficet – ChemoCentryx (Kendle)
Protocol CL004_282 A Multinational Double-Blind, Placebo-Controlled, Parallel Group Study to Evaluate the Efficacy and Safety of CCX282-B in Subjects with Moderate to Severe Crohn’s Disease
2004-6
CDP – Celltech (ICON)
Precise 1: Protocol CDP870-031 - A Phase III multi-national, multi-centre, double-blind placebo-controlled parallel group, 26 week study to assess the safety and efficacy of the humanised anti-TNF PEG conjugate, CDP870 400 mg sc, (dosed at Weeks 0, 2, 4 then 4-weekly to Week 24), in the treatment of patients with active Crohn's Disease
Precise 3: Protocol CDP870-033 A Phase III multi-national, multi-centre, open-label, 52 week safety study to assess the safety of chronic therapy with the humanised anti-TNF PEG conjugate, CDP870 400 mg sc, (dosed 4-weekly to Week 50), in the treatment of patients with active Crohn's Disease who have previously completed studies CDP870-031 or CDP870-032
Precise 4: Protocol CDP870-034 - A Phase III multi-national, multi-centre, open label, 52 week safety study to assess the safety of re-exposure after a variable interval and subsequent chronic therapy with the humanised anti-TNF PEG conjugate, CDP870 400 mg sc, (dosed at Weeks 0, 2, 4 then 4-weekly to Week 48), in the treatment of patients with active Crohn's Disease who have previously been withdrawn from studies CDP870-031 or CDP870-032
2005-6
Leukine – Schering (Schering/Novotech)
Protocol 308380 Phase 3 Randomized, Double-Blind, Placebo Controlled Induction Study of Sargramostim (Leukine) in Patients with Active Crohn’s Disease
2005-6
Leukine – Schering (Schering/Novotech)
Protocol 307340 Open-label Trial of Leukine (Sargramostim), A Recombinant Human Granolocyte-Macrophage Colony Stimulating Factor (GM-CSF), in Active Crohn’s Disease
2008
TSO – Asphelia (Novotech)
Protocol Number ASP1002-CD-201 A randomised, double-blind, placebo-controlled trial of ASP1002 (Trichuris suis ova [TSO]) therapy for moderately active Crohn's Disease.